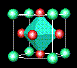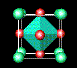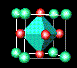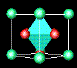
![]() ¶Linde Zeolite-A (LZA) is one of the most important zeolites;
it is harmless to the environment
and is used in washing powders to remove calcium and magnesium ions, which would otherwise be
precipitated from 'hard' water. Over 700 million tons, worth hundreds of millions of dollars,
are manufactured each year. In this zeolite, some of the tetrahedral silicon sites (green) are
occupied instead by aluminium (purple); aluminium oxide is another common constituant of sand.
¶Linde Zeolite-A (LZA) is one of the most important zeolites;
it is harmless to the environment
and is used in washing powders to remove calcium and magnesium ions, which would otherwise be
precipitated from 'hard' water. Over 700 million tons, worth hundreds of millions of dollars,
are manufactured each year. In this zeolite, some of the tetrahedral silicon sites (green) are
occupied instead by aluminium (purple); aluminium oxide is another common constituant of sand.
![]() Instead of drawing the SiO4 and AlO4 tetrahedrae, we can understand the architecture of zeolites
simply by connecting up the Si and Al atoms; this shows the
¶Zeolite-A frame-work structure, and emphasises
the different cavities and channels. You can learn more about zeolites from the
Zeolite Atlas
at ETH-Zurich, which provided this frame-work drawing, and you can generate your own 3D VRML drawings
of other structures from the
ILL Zeolite page.
Instead of drawing the SiO4 and AlO4 tetrahedrae, we can understand the architecture of zeolites
simply by connecting up the Si and Al atoms; this shows the
¶Zeolite-A frame-work structure, and emphasises
the different cavities and channels. You can learn more about zeolites from the
Zeolite Atlas
at ETH-Zurich, which provided this frame-work drawing, and you can generate your own 3D VRML drawings
of other structures from the
ILL Zeolite page.
The mineral world is full of beautiful naturally occuring structures, many
of which man has only recently learned to synthesize. Lets look at some
more examples from nature: gemstones & minerals.